Family Cancer History Risk Assessment Tool
Understand Your Cancer Risk
This tool helps you assess your potential cancer risk based on family history. It's not a medical diagnosis or substitute for professional genetic counseling.
Your Risk Assessment
Understanding cancer genetics can feel like navigating a maze of DNA letters, risk charts, and medical jargon. The good news? You don’t need a PhD to grasp the basics. This guide walks you through why genes matter in cancer, how testing works, and what steps you can take right now.
Why Genetics Matters in Cancer
Cancer genetics studies how changes in our DNA influence the development and spread of cancer. When a cell’s DNA gets altered, the normal checks that keep growth in line can fail, leading to uncontrolled division. Some mutations are random-called somatic mutation-while others are inherited from a parent, known as germline mutation. Both types can tip the balance toward cancer, but hereditary mutations often affect families across generations.
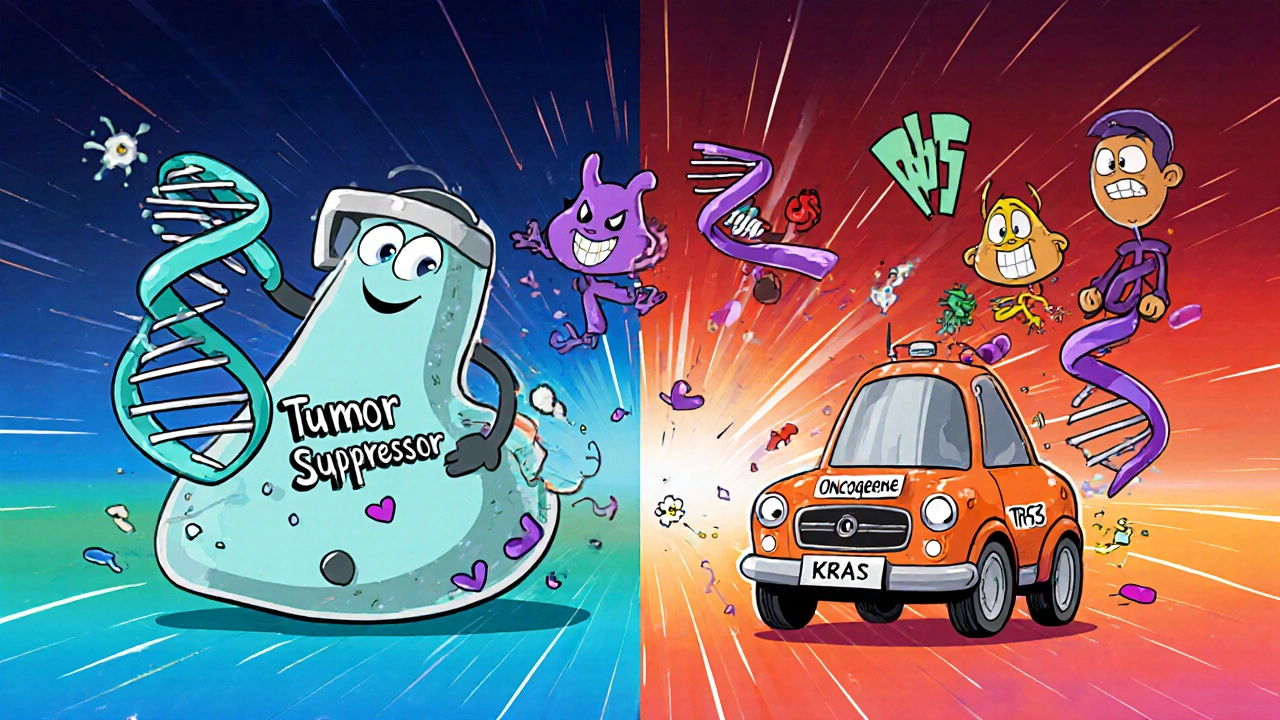
How Genetic Mutations Trigger Cancer
DNA is a set of instructions for building proteins. Certain proteins act as brakes (tumor suppressors) or accelerators (oncogenes) for cell growth. When a mutation disables a brake or flips an accelerator on, cells can start multiplying without restraint.
- Loss‑of‑function mutations in tumor‑suppressor genes (e.g., TP53) remove the cell’s ability to repair DNA or trigger cell death.
- Gain‑of‑function mutations in oncogenes (e.g., KRAS) give cells a permanent growth signal.
Most cancers involve a combination of both-multiple genetic hits accumulate over years before a tumor becomes clinically detectable.
Hereditary vs. Sporadic Cancers
Not all cancers are inherited, but a significant minority are. Below is a quick side‑by‑side look at the two categories.
| Feature | Hereditary (Germline) | Spontaneous (Somatic) |
|---|---|---|
| Source of mutation | Inherited DNA change present in every cell | Acquired change in a single cell during life |
| Typical age of onset | Earlier, often before 50 | Later, usually after 60 |
| Family history | Strong pattern across generations | Usually absent |
| Common genes | BRCA1/2, TP53, MLH1, MSH2 | Varies; may involve any gene |
| Screening recommendations | Intensive, earlier, sometimes MRI or colonoscopy | Standard population‑based guidelines |
Major Cancer‑Related Genes to Know
Below are the handful of genes that show up most often in discussions about inherited cancer risk.
- BRCA1 gene - Mutations raise breast and ovarian cancer risk up to 85%.
- BRCA2 gene - Similar to BRCA1 but also linked to pancreatic and prostate cancers.
- TP53 gene - Known as the “guardian of the genome,” germline changes cause Li‑Fraumeni syndrome, a predisposition to many tumor types.
- Lynch syndrome - A group of mutations (MLH1, MSH2, MSH6, PMS2) that dramatically increase colorectal and endometrial cancer risk.
- APC gene - Mutations lead to familial adenomatous polyposis, a condition that can cause hundreds of colon polyps.
Knowing whether you carry one of these mutations helps doctors personalize screening and prevention.
Genetic Testing: What, When, How
Genetic testing isn’t a one‑size‑fits‑all. The process typically follows three steps:
- Risk assessment - A doctor or genetic counselor reviews personal and family history.
- Sample collection - Usually a blood draw or cheek swab.
- Laboratory analysis - DNA sequencing looks for pathogenic variants in a panel of cancer‑related genes.
Tests fall into two categories:
- Diagnostic - Used after a cancer diagnosis to guide treatment (e.g., testing tumor tissue for somatic mutations).
- Predictive - Performed on healthy people with a strong family history to estimate future risk.
Results usually come with a report that categorizes variants as pathogenic, likely pathogenic, uncertain significance, likely benign, or benign.
Benefits of Knowing Your Genetic Risk
Having a clear genetic picture can change the game in several ways:
- Targeted screening - Earlier mammograms, colonoscopies, or MRI scans can catch tumors when they’re most treatable.
- Risk‑reduction strategies - Some choose prophylactic surgery (e.g., mastectomy for BRCA carriers) or chemoprevention.
- Informed family planning - Knowledge can guide decisions about having children and prenatal testing.
- Personalized therapy - Certain germline or somatic mutations qualify patients for targeted drugs or clinical trials.
Common Misconceptions
Even with growing awareness, myths linger:
- Myth: “If I test negative, I’m safe.” - A negative result only rules out the specific genes tested; other risks still exist.
- Myth: “Genetic testing is only for people with cancer.” - Predictive testing helps many at‑risk individuals before any disease appears.
- Myth: “Insurance will deny coverage.” - In many countries, including New Zealand, laws protect against discrimination for verified genetic tests.

Steps You Can Take Today
Don’t let the information overload stop you. Here’s a simple checklist:
- Write down any cancers diagnosed in your immediate family, including ages at diagnosis.
- Schedule a visit with a primary‑care physician and ask for a referral to a genetic counselor.
- Research reputable testing labs (e.g., labs accredited by ISO 15189).
- If a test is recommended, discuss possible outcomes and next steps before signing consent.
- Share relevant results with close relatives so they can consider testing too.
Frequently Asked Questions
Frequently Asked Questions
What is the difference between somatic and germline mutations?
Somatic mutations occur in a single cell during a person’s lifetime and are not inherited. Germline mutations are present in every cell from birth and can be passed to offspring.
Should everyone get genetic testing for cancer?
Testing is most useful for people with a strong family history, early‑onset cancers, or specific ethnic backgrounds with known founder mutations. Routine testing for the general population isn’t yet standard practice.
Can lifestyle changes offset a high genetic risk?
Yes. Even with a pathogenic mutation, avoiding tobacco, maintaining a healthy weight, exercising regularly, and limiting alcohol can lower overall risk and improve outcomes.
How accurate are current DNA tests?
Modern next‑generation sequencing can detect most known pathogenic variants with >99% accuracy. However, some regions of the genome are harder to read, and rare variants may be classified as “uncertain significance.”
What role does a genetic counselor play?
A counselor reviews your personal and family history, explains testing options, helps interpret results, and guides you through medical and emotional decision‑making.
Key Takeaways
- Cancer genetics links specific DNA changes to cancer risk and treatment options.
- Both inherited (germline) and acquired (somatic) mutations can drive tumor growth.
- Genes like BRCA1/2, TP53, and those involved in Lynch syndrome are the most common hereditary culprits.
- Genetic testing, paired with counseling, helps personalize screening, prevention, and therapy.
- Take action: map your family history, consult a professional, and consider testing if risk factors are present.





Emma Williams
October 18, 2025 AT 17:14Thanks for breaking it down – genetics can feel overwhelming, but you’ve made it clear. I especially liked the simple checklist at the end, it’s a great first step for anyone. Looking forward to sharing this with my family.
Stephanie Zaragoza
October 20, 2025 AT 02:18While the overview is commendable, precise clarification is required: the distinction between somatic and germline mutations must emphasize that somatic alterations arise post‑zygotically and are not heritable, whereas germline variants are present in every cell from conception; consequently, only germline mutations bear relevance to familial risk assessment. Moreover, the table could benefit from including penetrance percentages for each gene, as this quantifies the probability that a carrier will develop cancer. Finally, be explicit about the limitations of current next‑generation sequencing technologies, particularly regarding coverage gaps in GC‑rich regions.
James Mali
October 21, 2025 AT 11:22Sounds legit.
Janet Morales
October 22, 2025 AT 20:26I get what you’re saying, but the article already does a decent job without drowning the reader in lab‑room jargon. Not everyone needs a PhD‑level breakdown before they can act on their own health.
Tracy O'Keeffe
October 24, 2025 AT 05:30Honestly, the whole “keep it simple” narrative is a bit naïve; when you’re dealing with oncogenes like KRAS and tumor suppressors such as TP53, we’re navigating a molecular labyrinth that deserves nuanced discourse. The author’s omission of epigenetic modifiers, for example, borders on intellectual laziness.
Linda A
October 25, 2025 AT 14:34Genetics is more than just a list of scary gene names.
It’s a story about how tiny changes in our DNA can tip the balance between health and disease.
When a mutation knocks out a tumor suppressor, the cell loses an important safety check.
Conversely, a gain‑of‑function mutation in an oncogene acts like a stuck accelerator.
Most cancers, however, are the result of several “hits” accumulating over many years.
That’s why family history matters – inherited mutations give you a head start.
But having a pathogenic variant doesn’t guarantee you’ll get cancer, it just raises the odds.
Lifestyle choices such as quitting smoking, exercising, and eating well can still shift those odds in your favor.
Knowing your genetic risk also opens doors to targeted screening, like earlier colonoscopies for Lynch syndrome carriers.
In some cases, doctors even offer prophylactic surgeries to dramatically cut risk.
Genetic testing itself isn’t a crystal ball; the results come with categories ranging from benign to uncertain significance.
An “uncertain significance” finding can be frustrating, yet it often reflects our still‑growing knowledge base.
The key is to pair testing with counseling so you understand what the numbers really mean.
Ultimately, the decision to test should be guided by personal and familial context, not by hype.
So, keep the conversation open with your relatives and health team, and use the information as a tool, not a verdict.
Joe Moore
October 26, 2025 AT 22:38All that science talk is fine, but don’t forget the big picture: the pharma giants and data brokers are quietly selling your DNA to the highest bidder, and the “personalized medicine” hype is just a front for profit‑driven trials that rarely benefit the average patient.
Ayla Stewart
October 28, 2025 AT 07:42While it’s understandable to be wary, there are strict regulations in many countries that limit how genetic data can be shared, and reputable labs follow those safeguards diligently.
Poornima Ganesan
October 29, 2025 AT 16:46Let’s set the record straight: the article correctly states that BRCA1/2 mutations raise breast cancer risk up to 85%, but it glosses over the fact that risk modifiers like reproductive history and hormonal exposure can further influence those numbers. Moreover, the statement that “insurance will deny coverage” is not universally true; many jurisdictions have legislation protecting against genetic discrimination, though loopholes still exist. Finally, remember that a negative test result only rules out the specific panel examined – you could still carry rare variants not covered by standard tests. In short, genetics is a powerful tool, but it’s only one piece of a much larger puzzle.
Rajesh Singh
October 31, 2025 AT 01:50Exactly – wielding genetic knowledge responsibly is a moral imperative; we must ensure that the benefits of testing are shared equitably, not hoarded by elite institutions that prioritize profit over patient welfare.