Every year, thousands of people take medications that work exactly as they should. But for some, a drug that’s been on the market for years can suddenly show a new, serious risk. That’s not a flaw in the system-it’s how it’s supposed to work. The U.S. Food and Drug Administration (FDA) doesn’t wait for disasters to happen. It watches. It listens. And when it sees something dangerous, it speaks up. In 2025 alone, the FDA issued over 60 drug safety communications, each one a direct warning to patients and doctors about real, measurable risks tied to specific medicines.
What Are Drug Safety Communications, Really?
Drug Safety Communications (DSCs) aren’t press releases or rumors. They’re official, legally required notices from the FDA, published under Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act. These aren’t warnings about manufacturing mistakes or counterfeit pills. They’re about side effects that only show up after thousands-sometimes millions-of people have taken a drug for months or years. Clinical trials involve a few thousand patients over a few months. Real life? People take these drugs for decades. That’s where the hidden dangers hide.
For example, in July 2025, the FDA updated the labeling for every single opioid painkiller in the U.S. That’s 46 different products, from brand-name OxyContin to generic oxycodone tablets. The new labels now include hard numbers: if you take opioids for more than 90 days, you have about a 1 in 12 chance of developing an opioid use disorder. That’s not a guess. It’s based on two massive postmarket studies tracking over 1.2 million patients. These numbers changed how doctors talk to patients. No more saying, “Just don’t take more than prescribed.” Now they say, “Here’s the actual risk-1 in 12. Is that worth it for your pain?”
The Opioid Labeling Shift: A Turning Point
The July 2025 opioid update was the biggest safety action on pain meds since the 2016 CDC guidelines. But it didn’t come out of nowhere. The FDA required drugmakers to fund long-term studies after approval. The Opioid Postmarketing Consortium spent $187 million on two studies tracking addiction, overdose, and rare neurological damage like toxic leukoencephalopathy-a brain condition that can mimic stroke symptoms but only shows up after long-term opioid use. Now, every opioid label must include this risk, along with updated warnings about mixing opioids with gabapentinoids (like Neurontin) and the need to keep naloxone on hand.
Doctors are split. Some, like Dr. Michael Chen in California, say the numbers finally give them the tools to have honest conversations. Others, like Dr. Lisa Rodriguez in Texas, worry that patients with chronic pain who’ve been stable for years will be abruptly taken off meds because of fear, not facts. The U.S. Pain Foundation warned that without better access to physical therapy, nerve blocks, or cognitive behavioral therapy, these warnings could hurt more than help. The FDA didn’t mandate limits on prescriptions. It gave data. The rest is up to the clinician.
Other Major Alerts in 2025
Not all safety alerts are about opioids. In May 2025, the FDA added a warning to Zyrtec and Xyzal-two of the most common allergy meds-about a rare but serious risk of severe drowsiness, especially in children under 6. The agency didn’t pull the drugs. It just told parents and doctors: don’t give these to kids under 6 without checking with a provider first. About 25 million Americans use these drugs annually. The change was small, but critical.
Then there’s the ADHD stimulant warning from June 2025. Extended-release versions of methylphenidate (like Concerta) and amphetamines (like Adderall XR) can cause significant weight loss in young children. The FDA now requires doctors to check weight at baseline and every three months for kids under 6. About 9.4 million U.S. children are prescribed ADHD meds. That’s a lot of growth charts being monitored more closely.
And then there’s the flip side: clozapine. In August 2025, the FDA removed its Risk Evaluation and Mitigation Strategy (REMS)-a strict monitoring program that required monthly blood tests-for this antipsychotic. Why? Because over 30 years of real-world use showed the risk of life-threatening low white blood cell counts dropped significantly with better screening and patient selection. It’s rare for the FDA to reduce restrictions. This move means fewer clinic visits and lower costs for patients who’ve been stable for years.
What About Vaccines and Biologics?
Even vaccines aren’t immune to post-market scrutiny. In June 2025, the FDA updated the warnings for Pfizer’s Comirnaty and Moderna’s Spikevax after reviewing over 1.1 million cases of myocarditis and pericarditis reported to VAERS. The data showed a clear pattern: males aged 12 to 29 had the highest risk-1,195 cases per million second doses. The warning didn’t say “don’t vaccinate.” It said: “Know the signs-chest pain, shortness of breath, rapid heartbeat-and get checked if they appear within a week after the shot.”
Biologics are another frontier. In July 2025, the FDA began investigating Sarepta’s AAVrh74 gene therapy for Duchenne muscular dystrophy after reports of liver toxicity and sudden muscle weakness. These are cutting-edge, one-time treatments costing over $3 million per patient. The safety signals are rare, but the stakes are high. The FDA’s Sentinel Initiative, which tracks health records from 300 million Americans, is now being used to spot patterns like this faster than ever before.
Leqembi and the New Standard for Alzheimer’s Drugs
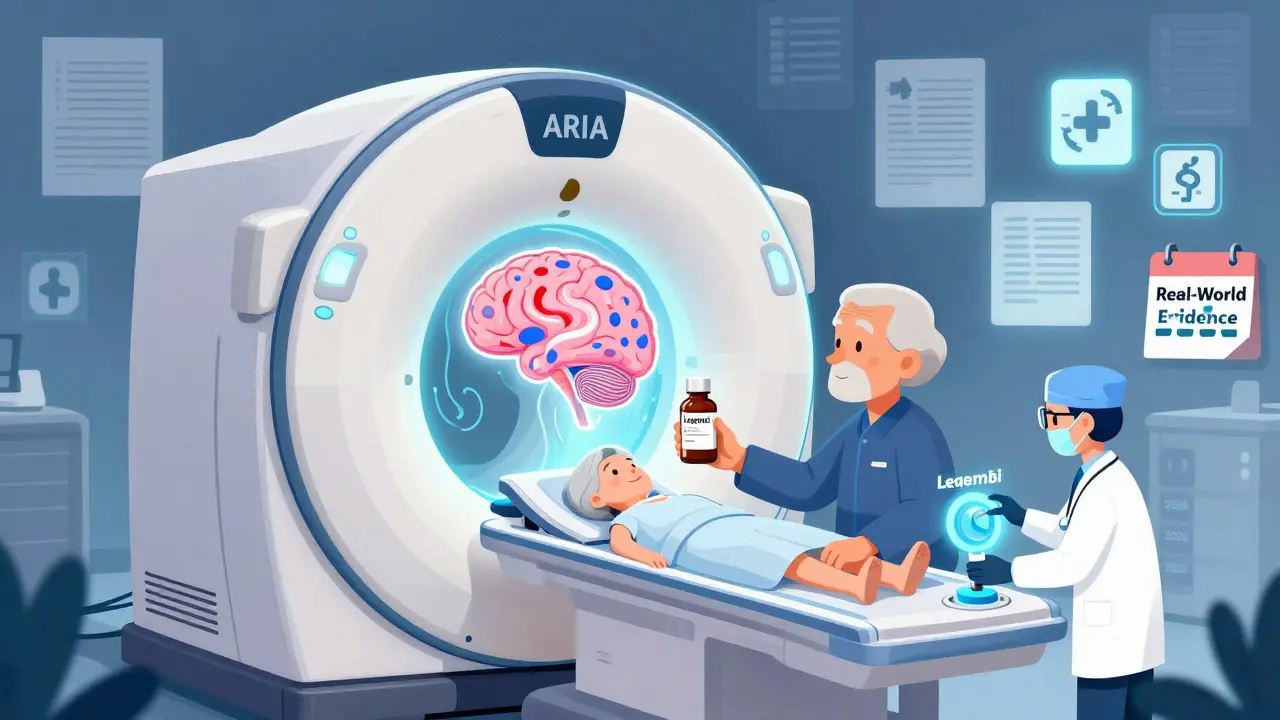
The most unusual alert came in August 2025: the FDA required MRI scans for patients taking Leqembi, the first Alzheimer’s drug shown to slow cognitive decline. Why MRIs? Because 274 cases of brain swelling or bleeding-called amyloid-related imaging abnormalities, or ARIA-were found in the first year of use. The FDA didn’t pull the drug. It said: “If you’re going to use this, you need to get an MRI before starting, then again at 5 and 14 months.” That’s a big ask. MRIs cost hundreds of dollars. Not every clinic has the capacity. But for patients and families desperate for any slowing of decline, it’s a trade-off they’re willing to make.
What Should You Do If You’re on a Medication?
Don’t panic. Don’t stop taking your meds without talking to your doctor. But do stay informed. Here’s what to do:
- Check your pill bottle. Is there a Medication Guide? It’s a small paper insert that comes with your prescription. If you don’t have one, ask your pharmacist for the latest version.
- Know your drug’s name. Generic and brand names can be confusing. Use Drugs.com or the FDA’s website to look up your exact medication.
- Ask your doctor: “Has there been any new safety info on this drug since I started taking it?”
- Watch for new symptoms. If you feel something off-unusual fatigue, chest pain, confusion, sudden weight loss-don’t assume it’s aging or stress. Call your provider.
- Use the FDA’s Drug Safety Communications page. It’s updated weekly. You don’t need to be a doctor to read it.
Pharmacies now offer free Medication Guides in 18 languages. The FDA also provides free clinician fact sheets and accredited training modules for doctors. You don’t have to hunt for this info-it’s there.
Why This Matters for Everyone
Drug safety isn’t just about bad pills. It’s about trust. When the FDA acts, it’s because the system worked: a patient reported a symptom, a doctor flagged it, the data piled up, and the agency responded. It’s slow. It’s messy. But it’s real. The opioid crisis taught us what happens when we ignore early warnings. Now, the FDA is trying to catch problems before they become epidemics.
The cost? The pharmaceutical industry spent $187 million just on opioid studies. Postmarketing safety budgets rose 28.5% from 2020 to 2024. That’s money that could’ve gone to new drugs. But it’s also money that’s saving lives. The FDA’s goal is to issue safety alerts within 30 days of confirming a risk-down from 60 to 90 days. That’s faster. That’s better.
You don’t need to be a scientist to understand this: every drug has risks. But with better data, better communication, and better monitoring, those risks become manageable-not mysterious.
Are medication recalls common?
True recalls-where a drug is pulled from shelves-are rare. Most safety actions are labeling updates, not removals. In 2025, only 3 medications were fully recalled by the FDA. The rest were safety communications: updated warnings, new monitoring rules, or revised dosing advice. A recall usually means a manufacturing defect, contamination, or mislabeling-not a newly discovered side effect.
Can I trust generic drugs if there’s a safety alert on the brand name?
Yes. Safety alerts apply to the active ingredient, not the brand. If the FDA warns about oxycodone, it applies to OxyContin, generic oxycodone, and any other product with that same chemical. Generics must meet the same safety and effectiveness standards as brand-name drugs. The alert isn’t about quality-it’s about how the body reacts to the drug over time.
What should I do if I think my medication is causing a side effect?
First, don’t stop taking it without talking to your doctor. Then, report it. The FDA’s MedWatch program lets patients and doctors report side effects online at fda.gov/medwatch. Even one report can help build the evidence needed for a safety update. Thousands of reports are needed before the FDA acts-but every one counts.
Do these safety alerts mean my drug is unsafe?
No. It means the drug has risks-and now we know them better. All medications have risks. The goal isn’t to eliminate them, but to understand them. For example, Leqembi has serious brain risks, but it’s also the only drug proven to slow Alzheimer’s progression. The choice isn’t “safe” vs. “unsafe.” It’s “is the benefit worth the risk-for me?” That’s a conversation to have with your doctor.
How often does the FDA update drug safety info?
The FDA releases new Drug Safety Communications weekly. You can sign up for email alerts on their website. Between 2020 and 2024, the number of safety alerts rose by 45%. That’s not because drugs are getting more dangerous-it’s because we’re watching more closely. Better data systems, more patient reports, and smarter analysis mean we catch problems faster.
What’s Next?
The FDA’s 2026-2030 plan includes a big shift: requiring real-world evidence for all drugs with black box warnings-the strongest safety alerts. That means if you’re prescribed a drug with a black box warning, your doctor may be asked to report outcomes over time. It’s a step toward personalized safety. It’s also a sign that the system is evolving from “watch and react” to “predict and prevent.”
For patients, that means more responsibility. More questions. More conversations. But also more power. You’re not just a recipient of a prescription-you’re part of the safety network. Your voice, your reports, your questions matter. And now, more than ever, the system is listening.




Mario Bros
January 10, 2026 AT 06:38Just took my Adderall XR and checked my kid’s growth chart-yikes. Glad the FDA’s on it. 👍
McCarthy Halverson
January 10, 2026 AT 13:47Label updates aren’t recalls. Stop panicking. Talk to your doc. Simple.
Lisa Cozad
January 11, 2026 AT 12:39I work in pharmacy and we’ve been handing out updated Medication Guides like candy since July. Patients are actually reading them now. Small win. The FDA’s finally making info accessible instead of burying it in legalese.
Saumya Roy Chaudhuri
January 12, 2026 AT 04:26Let me tell you something about clozapine-my cousin was on it for 12 years and the monthly blood draws were torture. The FDA removing the REMS? That’s not progress, that’s laziness. They’re cutting corners because hospitals can’t afford the monitoring anymore. And now they want us to trust them? Please.
chandra tan
January 12, 2026 AT 13:17In India we get generic opioids but no one talks about the risks. I showed my uncle the FDA update-he said, ‘If it’s cheaper, it’s better.’ That’s the problem. Education doesn’t reach everyone. The FDA’s doing great work-but it’s not global.
Bradford Beardall
January 13, 2026 AT 19:47Remember when people thought SSRIs caused suicide? Now we know it’s more nuanced. Same thing here. The FDA isn’t saying ‘stop taking this’-it’s saying ‘know the signs.’ That’s science in action. We used to rely on anecdote. Now we have data from 300 million people. That’s revolutionary.
Dwayne Dickson
January 15, 2026 AT 19:42The notion that postmarketing surveillance is ‘how the system is supposed to work’ is a masterclass in institutional self-congratulation. The opioid crisis was not a failure of detection-it was a failure of will. $187 million spent on studies after 20 years of denial? That’s not vigilance, that’s damage control dressed in white coats. And now we’re supposed to applaud the FDA for finally acknowledging what community doctors have been screaming about since 2010? The only thing that’s changed is the PR department’s vocabulary. The system didn’t work-it was broken, and it took a generation of dead bodies to fix the label.
Let’s not confuse transparency with accountability. You don’t fix a culture of corporate capture by issuing a PDF. You fix it by prosecuting the executives who suppressed early signals. You fix it by making postmarket surveillance mandatory-not optional. You fix it by forcing manufacturers to fund independent research, not their own cherry-picked cohorts. The FDA’s role is not to be a librarian of risk-it’s to be a shield against predatory profit. And right now? It’s just a very well-funded PR firm with a badge.
And don’t get me started on Leqembi. You want patients to get MRIs every five months? Fine. But who pays? Medicare won’t cover it. Private insurers won’t budge. So the only people who benefit are those who can afford $800 scans on top of a $26,000-a-year drug. That’s not medicine. That’s a luxury subscription service for the dying wealthy. And you call this ‘progress’?
Real safety isn’t about updating labels. It’s about dismantling the incentives that let drugmakers gamble with human lives for quarterly earnings. Until then, these communications are just elegies written in bureaucratic font.
Faith Edwards
January 16, 2026 AT 00:56Oh, how delightfully quaint-the FDA, that bastion of unimpeachable integrity, finally deigns to inform the plebeian masses that opioids can be addictive. How novel. One assumes the pharmaceutical titans who funded the very studies that proved these risks were also the ones who lobbied to delay the label changes for nearly a decade. How noble. How virtuous. How utterly, profoundly, theatrically *late*. And yet, we are expected to be grateful? To applaud the slow drip of truth from a dam built on shareholder dividends? How gauche of us to expect moral clarity from institutions that monetize human suffering as a line item in their annual report.
And now, we are told to ‘check your pill bottle’-as if the Medication Guide, printed in 6-point font on recycled paper, is some sacred text. How charming. How utterly infantilizing. You think a patient with chronic pain, on six different medications, and a 12-hour workday, is going to parse the FDA’s latest 47-page PDF on ARIA risks? No. They’re going to Google it at 2 a.m., find a Reddit thread written by someone who ‘knows a guy,’ and then decide whether to keep taking the drug that lets them breathe. That’s not healthcare. That’s survival theater.
And let us not forget the sacred cow of generics-‘they’re the same!’ they cry. As if chemical equivalence negates the chasm between a $2 pill made in a factory with no oversight and a $12 pill made under the watchful eye of a compliance officer who has been paid to look the other way. The FDA doesn’t regulate quality-it regulates paperwork. And now, we are to trust the same institution that allowed fentanyl-laced counterfeit pills to flood the market for years? The same institution that gave a pass to Purdue Pharma for a decade? The same institution that still allows drugmakers to market off-label uses with a wink and a subpoena?
So yes, by all means, update the labels. But don’t mistake the ink for justice. Don’t confuse the warning with the wound. The real tragedy isn’t the side effect-it’s the fact that we’ve normalized the idea that human life is a cost center in a profit-driven system. And until we stop treating patients like data points and corporations like saints, no amount of ‘safety communications’ will ever be enough.
Jay Amparo
January 16, 2026 AT 15:08As someone from India who’s seen both sides-cheap generics and expensive biologics-I just want to say: this post is a lifeline. In my village, people take pills without knowing what’s in them. My aunt took Zyrtec for her kid and didn’t know it could cause drowsiness. She almost missed the signs until the school called. This kind of info needs to be translated, shared, shouted from rooftops. The FDA’s doing the right thing-but it’s only half the battle. We need community health workers, WhatsApp groups, radio broadcasts in 18 languages. Knowledge is power, but only if it reaches the people who need it most. Let’s not just update labels-let’s update access.